Ten years of Uncommon Pediatric Illness Designation: impression on drug approvals
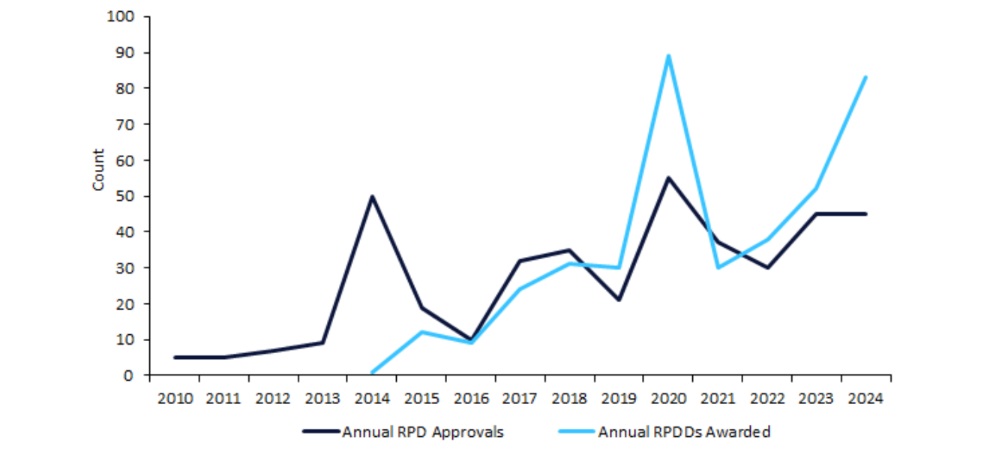
As a consequence of considerations surrounding its effectiveness, the US Meals and Drug Administration (FDA) ended the Uncommon Pediatric Illness Designation (RPDD) programme in late 2024. Following this programme’s introduction in 2014, the FDA oversaw a surge in drug approvals for uncommon pediatric problems (RPDs). From 2016 to 2024, a optimistic pattern in RPDDs awarded was mirrored by a normal improve in RPD approvals. These findings counsel that the implementation of RPDDs positively impacted the RPD market. Nevertheless, their final impression has acquired scrutiny.
Given the small affected person populations and restricted monetary incentives, RPDs have been traditionally neglected in drug improvement. In July 2012, the FDA launched the RPDD. This designation was awarded to medicine that have been being developed for uncommon and critical illnesses that primarily have an effect on people youthful than 18. The RPDD supplied monetary incentives to pharma firms creating these therapies. If a drug with RPDD was subsequently authorised, the corporate acquired a Precedence Overview voucher. This voucher supplied a streamlined and prioritised FDA evaluate for a future drug or may very well be offered for as a lot as $350m.
As a consequence of considerations over its effectiveness, the US Congress didn’t renew the RPDD programme and started to shut the programme in late 2024. Determine 1 (above) highlights traits in RPD approvals from 2010 to 2024, alongside annual RPDDs awarded by the FDA, which commenced in 2014.
Between 2010 and 2013, annual FDA approvals for RPDs remained comparatively low and steady, at a mean of 6.5 per 12 months.
In 2014, the FDA started awarding RPDDs. This identical 12 months, the FDA awarded 50 approvals for RPDs, at a five-fold improve from 2013. Nevertheless, solely one of many 50 RPD approvals in 2014 was linked to an RPDD. Thus, the 2014 surge in RPD approvals was as a consequence of a broader FDA initiative to prioritise paediatric illnesses, versus the RPDD programme having a direct impression.
In 2016, solely ten RPDs have been authorised by the FDA. Regardless of numerous peaks and troughs between 2016 to 2024, this era noticed an total optimistic pattern for annual RPD approvals, rising at a compound annual development charge (CAGR) of 16.2%. A peak of RPD approvals got here in 2020, with the FDA handing out 55 approvals. Over this era, traits in RPDD awarding have been equally optimistic, at a CAGR of 24.9%. Like RPD approvals, 2020 was a peak 12 months for RPDDs with 89 designations awarded, an virtually three-fold improve from the 30 designations awarded in 2019. This paralleled development means that the RPDD programme could have performed a reinforcing function within the elevated trade give attention to uncommon paediatric therapies.
These findings counsel that the FDA started putting considerably larger emphasis on RPD drug improvement in 2014 via the introduction of the RPDD programme and a surge in RPD approvals. Nevertheless, the direct impression of RPDDs available on the market stays unclear. However, will increase in each RPDDs awarded and RPD approvals from 2016 counsel that the FDA’s focus continued, with the providing of monetary incentives probably stimulating enhanced efforts from drug builders. Because the RPDD programme closes down, the long run RPD panorama stays unsure. New coverage efforts have to be launched to make sure the pattern of accelerating RPD therapies and approvals continues for years to return.






