Strong tumours: CAR-T therapies’ Waterloo
CAR-Ts are revolutionising the therapy of blood cancers equivalent to B-cell acute lymphocytic leukaemia. Nonetheless, their success has not but prolonged to the realm of strong tumours, as no CAR-T remedy has superior past Part II in one among these indications. Overcoming present limitations and increasing the attain of CAR-T therapeutics might unlock promising new therapy prospects for strong tumour sufferers.
CAR-T therapeutics are a number one kind of T-cell immunotherapy, accounting for over half of the approvals within the oncology cell and gene remedy panorama. This therapeutic modality includes genetically engineering autologous or allogeneic T-cells to specific a chimeric antigen receptor in order that they actively recognise and destroy cancerous cells. In whole, 13 CAR-T therapies have acquired regulatory authorisation, together with the 2025 approval of Immuneel Therapeutics’ Qartemi (varnimcabtagene autoleucel), in response to the latest report, ‘T-Cell Immunotherapy Panorama: Complete Evaluation of Present Medicine and Dynamics’. By way of gross sales, Gilead’s Yescarta is the main CAR-T. Having acquired FDA approval in 2017, this product generated $1.6bn worldwide in 2024. Much like all different CAR-Ts available on the market, Yescarta treats blood cancers and is redefining therapy paradigms for indications equivalent to B-cell acute lymphocytic leukaemia.
Strong tumours characterize roughly 90% of all grownup human cancers, together with breast, lung, and pancreatic most cancers. Regardless of vital success within the area of blood cancers, CAR-Ts haven’t skilled an identical degree of success in strong tumours, as there have been no CAR-T approvals in associated indications. Thus far, essentially the most superior stage for a CAR-T in a strong tumour is Part II (Determine 1).
Determine 1: Growth stage share break up for energetic CAR-Ts in strong tumour indications
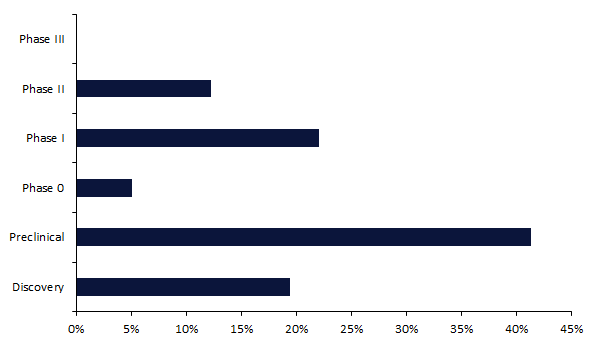
An energetic pipeline stage refers back to the improvement phases: Discovery, Preclinical, Part 0, Part I, Part II, and Part III.
At present, over 650 CAR-Ts are in energetic improvement for a strong tumour indication. Over 40% of those are within the preclinical stage, and solely 80 (12%) merchandise are in essentially the most superior stage, Part II.
There are a number of challenges related to growing CAR-Ts to deal with strong tumours. The heterogeneity of strong tumours and the absence of particular tumour antigens, alongside the immunosuppressive tumour microenvironment, make it tough for CAR-Ts to infiltrate and persist inside the tumour. These challenges restrict the efficacy of CAR-Ts when treating strong tumours, which is confirmed by GlobalData’s Medicine Intelligence database, as no CAR-T has ever accomplished a Part II trial and entered Part III for a strong tumour.
Bristol Myers Squibb (BMS), which is a frontrunner within the CAR-T panorama, accounts for 2 of the 13 marketed merchandise: Abecma (idecabtagene vicleucel) and Breyanzi (lisocabtagene maraleucel). It’s also a joint chief within the strong tumour panorama, with 12 CAR-Ts in improvement. BMS is seeking to prolong the label for Breyanzi to strong tumours, as this product is at present in Part II for main and secondary central nervous system (CNS) lymphoma.
Elsewhere, China-based Shenzhen Geno-Immune Medical Institute is matching BMS within the strong tumour pipeline, with 12 CAR-Ts in improvement. Nonetheless, Shenzhen’s portfolio is extra superior, with 11 Part II merchandise, in comparison with just one for BMS; as such, Shenzhen might overtake BMS because the front-runner on this space.
CAR-Ts have demonstrated outstanding potential, however to this point, their success has remained confined to blood cancers with little success in different indications. Given the prevalence of strong tumours, overcoming the constraints of CAR-Ts in these indications is essential, and as such, there’s a sturdy concentrate on the CAR-T market on this space. Success might rework most cancers therapy, offering new hope for sufferers and proving commercially useful for drugmakers.






