Olutasidenib improves survival for myelodysplastic syndrome
August 20, 2025
5 min learn
Key takeaways:
- Olutasidenib monotherapy or mixture remedy could assist sure sufferers with myelodysplastic syndrome obtain sturdy response.
- Many sufferers within the examine achieved transfusion independence.
Olutasidenib may dramatically prolong survival and scale back dependence on transfusions for sure sufferers with myelodysplastic syndrome, in keeping with outcomes of a section 1/2 investigation.
People with higher-risk illness and an IDH1 mutation who acquired olutasidenib (Rezlidhia, Rigel Prescription drugs) as monotherapy or as a part of mixture remedy had a median OS of greater than 2 years.

Information derived from Cortes JE, et al. Blood Adv. 2025;doi:10.1182/bloodadvances.2025016718.
Moreover, median length of remission lasted greater than 20 months and greater than 60% of sufferers achieved transfusion independence.

Justin M. Watts
“If you see a affected person with myelodysplastic syndrome with an IDH1 mutation, you need to instantly take into consideration an IDH1 inhibitor,” Justin M. Watts, MD, affiliate professor of medication within the division of hematology and chief of the leukemia part at Sylvester Complete Most cancers Heart, a part of the College of Miami Miller Faculty of Drugs, informed Healio.
Commonplace ‘has not modified’
Within the U.S., 4.5 per 100,000 people are identified with myelodysplastic syndrome (MDS) yearly, in keeping with examine background.
Threat rises considerably as folks become old (ages 70-79 years, 26.9 per 100,000).
Sufferers with low-risk illness are predominantly handled to enhance cytopenia. These with higher-risk MDS usually obtain remedy with a hypomethylating agent comparable to azacitidine or decitabine.
People with relapsed/refractory illness have a median OS round 6 months, in keeping with Watts.
“The usual of care has not modified in 30 years,” Watts stated of remedy with azacitidine. “It’s not a nasty drug, that’s why it’s permitted. It really works in some sufferers, however solely about 20% of sufferers within the entrance line have an actual good response and one other handful have some response, and the sturdiness is suspect.
“The difficulty with MDS is it doesn’t have as many targetable mutations as acute myeloid leukemia or another cancers, however IDH1 or IDH2 are notable exceptions,” he added. “When the affected person has that mutation, they are typically very depending on it.”
Roughly 3% to 4% of sufferers with MDS have an IDH1 mutation.
In 2022, the FDA permitted olutasidenib, an IDH1 inhibitor, for sure sufferers with AML primarily based on analysis by which Watts and colleagues participated.
That examine additionally enrolled people with MDS.
“One factor we seen in regards to the sufferers with AML who’ve very lengthy sturdiness of response and survival with olutasidenib is that they resemble extra of an MDS-like mutational sample,” he stated.
The investigation included 22 adults (median age, 74 years; vary, 59-87; 59% males) with intermediate- to very high-risk MDS and an IDH1 mutation, however no prior remedy with an IDH1 inhibitor.
Contributors may have newly identified (n = 7) or relapsed/refractory illness (n = 15).
They acquired both olutasidenib monotherapy (n = 6) or together with azacitidine (n = 16) primarily based on doctor advice.
After a section 1 dose-escalation stage, researchers really helpful a section 2 dose of 150 mg olutasidenib twice day by day for steady 28-day cycles no matter arm. Adults within the mixture cohort additionally acquired 75 mg/m² azacitidine day by day for 7 days.
Security and full remission served as major endpoints. General response charge, time to response, length of response, EFS, OS and transfusion independence (56 days after baseline with out transfusion) served as secondary endpoints.
‘Very robust survival numbers’
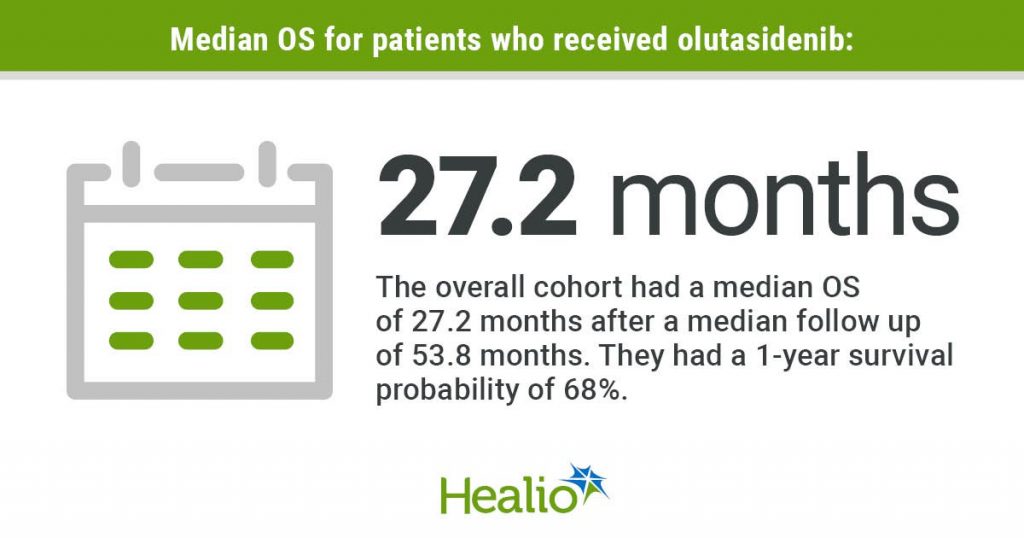
After a median follow-up of 53.8 months, the general cohort had an OS of 27.2 months (95% CI, 6-37) and a 1-year survival chance of 68% (95% CI, 45%-83%).
Sufferers within the mixture arm had a better median OS than those that received monotherapy (27.5 months vs. 14 months).
Newly-diagnosed individuals didn’t attain median OS and had 86% survival at 1 and a couple of years.
“Frontline sufferers did remarkably nicely,” Watts stated.
Sufferers with relapsed/refractory illness had a median OS of 16.3 months (95% CI, 3.1-36.6), 60% survival at 1 12 months and 43% at 2 years.
“These are very robust survival numbers,” Watts stated.
The general cohort had an ORR of 59%, full remission occurred in 27% of individuals and 32% achieved marrow full remission.
The general cohort had a median length of response of 20.5 months (95% CI, 4.9-not reached).
Amongst sufferers that had marrow full remission, 57% had hematologic enchancment in at the very least one lineage.
Within the monotherapy arm, solely three sufferers acquired the really helpful dose. Every of them achieved an entire remission, marrow full remission and hematologic enchancment.
Within the mixture group, individuals who had treatment-naïve illness had an ORR of 100% in contrast with 54.6% for individuals who had relapsed/refractory MDS. Moreover, 80% of full remissions within the arm had been newly identified sufferers.
Amongst individuals depending on purple blood cell transfusions, 62% had 56-day transfusion independence and 23% remained dependent.
“That’s one other robust signal of true organic exercise in MDS,” Watts stated. “If you see actual, sustained blood depend enchancment and transfusion independence, you recognize your drug’s working.”
All individuals skilled at the very least one treatment-emergent hostile occasion and 95% had a grade 3 or worse occasion.
The commonest any-grade treatment-emergent hostile occasions included fatigue (67% in monotherapy; 63% together), nausea (67%; 56%) and arthralgia (67%; 31%). A number of sufferers (56%) within the mixture group additionally skilled constipation.
Widespread grade 3 or worse treatment-emergent hostile occasions within the monotherapy arm included thrombocytopenia (33%) and neutropenia (33%). Essentially the most frequent within the mixture group included neutropenia (38%), febrile neutropenia (31%) and thrombocytopenia (25%).
“IDH inhibitors are top-of-the-line tolerated medication ever utilized in leukemia of any class,” Watts stated. “They’re very nicely tolerated, even in comparison with different kinase inhibitors. You don’t see plenty of pores and skin or GI toxicity or myelosuppression. They every have some idiosyncratic unintended effects which can be manageable. For olutasidenib, one factor that we did see in about 10% of sufferers was [elevated liver enzymes] of significance, and if that occurred, we needed to maintain after which resume the drug, generally at a decrease dose. It normally received higher, and sufferers have been in a position to proceed the examine.”
“Lastly, the category impact of differentiation syndrome happens in about 10% to fifteen% of sufferers with MDS or AML and requires shut monitoring however is manageable with commonplace supportive care,” he added.
On the finish of the examine, 13.6% of sufferers stayed on remedy.
Researchers acknowledged examine limitations, together with the small pattern dimension.
‘Commonsense commonplace of care’
Watts doesn’t anticipate a randomized section 3 trial for olutasidenib because of the restricted affected person inhabitants.
Nonetheless, the collective knowledge recommend olutasidenib or different IDH1 inhibitors ought to be a part of remedy for people with higher-risk illness and an IDH1 mutation.
It ought to be “commonsense commonplace of care,” Watts stated.
“The subsequent steps should not confirming anything in MDS or AML with single agent or with azacitidine,” he added. “It’s how will we treatment sufferers?”
Watts and colleagues have reported on one affected person who has been on olutasidenib for almost a decade and stays in remission, and she or he just isn’t alone.
“It’s a big minority,” he stated.
Combining IDH inhibitors with azacitidine and different brokers, comparable to venetoclax (Venclexta; AbbVie, Genentech), notably within the first-line setting, may benefit extra sufferers.
Different mutations could possibly be focused, too.
“We’re blessed to have all these new brokers in AML to mix, sequence and to play with to enhance outcomes in sufferers, all which have their very own particular person exercise,” Watts stated. “The way to finest mix all that to maximise treatment charge is what we’re attempting to do.”
References:
For extra info:
Justin M. Watts, MD, could be reached at jxw401@miami.edu.






