Aktiia will get FDA clearance for OTC cuffless blood strain monitor
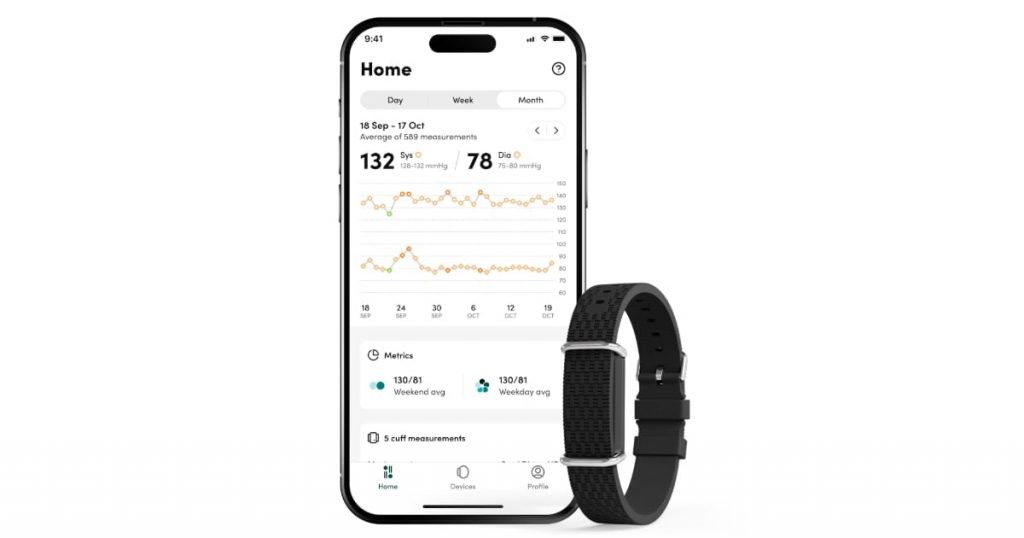

Healthcare know-how firm Aktiia has acquired FDA 510(okay) clearance for its over-the-counter cuffless blood strain monitor, G0 Blood Stress Monitoring System, often known as the Hilo Band.
Aktiia’s blood strain system, recognized below the Hilo model in Europe, is a wearable that makes use of an optical sensor to gather knowledge from one’s wrist. That knowledge is then despatched to the Hilo app and Hilo’s cloud server, the place algorithms estimate the wearer’s blood strain utilizing pulse wave evaluation.
The corporate says the evaluation examines the distinctive type of strain exerted by every heartbeat by way of the wearer’s blood vessels.
The wrist wearable repeatedly collects one’s blood strain all through the day, however does so solely when they’re nonetheless.
The product, which is already CE marked as a Class IIa medical gadget for monitoring blood strain in adults in Europe, shall be out there to U.S. customers in 2026.
“This milestone is deeply private for us as founders and profoundly necessary for everybody at Aktiia. From day one, our mission has been to deal with the worldwide hypertension disaster by way of know-how. With this FDA clearance, we’re one step nearer to realizing our imaginative and prescient: a future the place wholesome blood strain is accessible to all,” Dr. Josep Solà, cofounder and chief know-how officer at Aktiia, informed MobiHealthNews.
“What makes this second actually historic is not only the clearance itself, however the truth that the FDA has authorised the Aktiia gadget for over-the-counter use, no prescription required. It is a uncommon and demanding bar to fulfill, and a transparent sign that our know-how isn’t solely clinically validated however prepared for real-world, large-scale deployment.”
Dr. Mattia Bertschi, cofounder and CEO, informed MobiHealthNews that the FDA clearance helps the corporate develop its attain.
“This opens a brand new marketplace for us, however extra importantly, it confirms that Aktiia’s resolution is secure, intuitive, and designed for the individuals who want it most. We consider that is just the start, and that cuffless, wearable, and over-the-counter blood strain monitoring will quickly grow to be the worldwide commonplace,” Bertschi stated.
“We’re working day and evening to make sure that Hilo by Aktiia does not simply take part on this transformation… however leads it.”
THE LARGER TREND
In Could, Aktiia scored $42 million in an oversubscribed Sequence B funding spherical, bringing its complete elevate to greater than $100 million.
Final yr, Aktiia accomplished a CHF 27 million ($30 million) funding spherical and acquired CE mark in Europe for its calibration-free know-how CALFREE, which permits blood strain knowledge to be collected utilizing inputs from optical sensors generally present in smartwatches and cameras of economic smartphones.
The Swiss firm stated that approval of its CALFREE tech would allow it to supply integration of its medical-grade optical blood strain monitoring to third-party corporations creating shopper gadgets, comparable to smartwatches, smartphone cameras, and sensible bands.
In 2021, the corporate raised $17.5 million in Sequence A funding, and the yr earlier than, it secured $6.1 million (CHF 6 million) in funding.
The corporate, based in 2018, was a spin-out of CSEM, a Swiss analysis and growth heart.