1 in 6 sufferers in part 2 most cancers trials obtain therapies which might be in the end accepted
April 15, 2025
6 min learn
Key takeaways:
- One in six members in part 2 most cancers trials obtained a remedy that gained FDA approval.
- The outcomes display part 1 trials assist filter out unsafe or ineffective medicine, investigators concluded.
Jonathan Kimmelman, PhD, has printed a number of analysis papers and gotten into an inordinate variety of debates in regards to the scientific worth of part 1 trials.
“I’ve argued for a very long time that for part 1 trials, what justifies the chance [of patient participation] is just not the prospect of getting a therapeutic drug, however reasonably the scientific worth,” Kimmelman, James McGill Professor of Biomedical Ethics at McGill College in Montreal, instructed Healio.

Information derived from Ouimet C, et al. J Natl Most cancers Inst. 2025;doi:10.1093/jnci/djaf013.
“Whenever you design a part 1 research, it’s actually necessary that you’ve got a very good scientific rationale,” he added. “[It should be] designed and reported nicely so that you’re getting as a lot scientific worth as attainable, as a result of the affected person is basically getting a nontherapeutic drug. They’re being uncovered to danger with out the justification of the profit.”
Kimmelman and colleagues beforehand discovered only one.2% of sufferers who take part in part 1 most cancers trials obtain a drug that ultimately positive aspects FDA approval.
Conversely, therapies that advance to part 3 have already demonstrated the potential of scientific profit.
The last word worth of remedies evaluated in part 2 trials, nevertheless, had been much less clear.
Kimmelman and colleagues carried out a retrospective research to look at that problem. Outcomes confirmed roughly one in six sufferers who participated in part 2 most cancers trials obtained a remedy that ultimately garners FDA approval.
“It’s as much as a affected person to resolve how they wish to interpret these numbers,” Kimmelman mentioned. “Some sufferers might say, ‘One-in-six odds of getting a drug that’s going to be accepted for my indication is price taking the chance [or] price the additional burdens of taking part in a trial.’ Others will say, ‘Why would I do that? There’s a five-in-six likelihood I’m going to get an unsafe and/or ineffective drug after I go into the trial. What’s extra, if I’m in that fortunate one in six, there’s nonetheless solely a 25% or much less likelihood that I’m truly going to have a therapeutic response.”
Background, strategies
A number of printed research have evaluated the risk-benefit stability of part 1 most cancers trials.
Analysis has proven goal response charges sometimes vary from 3.8% to 13.2%, in response to research background. Life-threatening opposed occasions occurred at charges between 10% and 19%.
“There hasn’t been very a lot that’s centered on what I name ‘the forgotten center little one’ of drug growth, that are the part 2 trials,” Kimmelman mentioned. “There isn’t a lot knowledge on the market about danger and profit. … It’s a spot that we’ve had for a very very long time.”
Kimmelman and colleagues — together with Charlotte Ouimet, MSc, PhD candidate in experimental drugs at McGill College — aimed to handle that proof hole.
They recognized 1,154 eligible part 1, part 1/part 2, or part 2 trials initiated between Nov. 1, 2012, and Nov. 1, 2015. They then randomly sampled 400 of these trials, which included 25,002 members and assessed 332 medicine.
Nearly all of trials have been part 2 (57%), included sufferers with strong tumors (77%) and evaluated mixture therapies (57%). Lower than half (43%) included dose escalation cohorts.
The researchers assessed whether or not the drug, dose and indication evaluated in every trial had been FDA accepted inside 7.5 years, whether or not it obtained an off-label suggestion primarily based on Nationwide Complete Most cancers Community tips and whether or not it produced a considerable scientific profit primarily based on ESMO Magnitude of Scientific Profit Scale (MCBS).
The variety of members in part 2 trials to obtain an FDA-approved remedy served as the first endpoint.
Outcomes
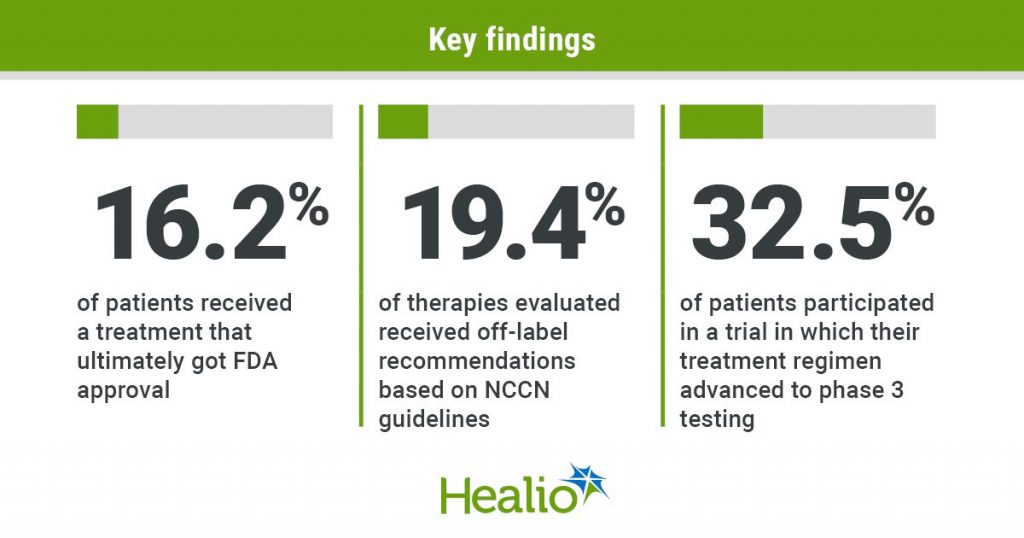
In all, 16.2% (95% CI, 10.3%-22.7%) of sufferers within the pattern group obtained certainly one of 71 remedy regimens that garnered FDA approval.
“We definitely anticipated to see the proportion enhance [from phase 1 trials], however I didn’t anticipate this diploma of enchancment,” Kimmelman mentioned. “I in all probability would have mentioned possibly one in 10, or possibly one in 15. I might not have predicted one in six.”
Outcomes confirmed 19.4% (95% CI, 14.1%-25.8%) of therapies evaluated obtained off-label suggestions primarily based on NCCN tips, and 9.3% (95% CI, 4.7%-14.6%) produced substantial scientific profit primarily based on ESMO-MCBS.
Practically one-third (32.5%; 95% CI, 26%-38.8%) of sufferers participated in a trial by which the remedy superior to part 3 testing.
Therapeutic response didn’t fluctuate considerably by trial sponsorship, drug part or trial traits.
Outcomes confirmed sufferers who obtained immunotherapy or participated in trials that used biomarker enrichment had a better chance of therapeutic profit. Nevertheless, the evaluation didn’t have enough statistical energy to scrupulously check that affiliation, Kimmelman mentioned.
“We are able to’t actually make conclusions from our knowledge alone,” he mentioned. “However should you see these traits alongside the literature, it reinforces the concept should you’re a affected person and you’ve got a alternative to enter a part 2 research the place you’ll be chosen primarily based on a biomarker vs. one the place you’re not, go for the research with the biomarker testing.”
Researchers acknowledged research limitations, together with use of historic part 2 trials — which can not present an correct illustration of present trials — in addition to the shortcoming to trace which trial traits had a better chance of approval and therapeutic profit.
“I can’t actually say whether or not one in six is nice or not,” Kimmelman mentioned. “What I feel is encouraging is, if we actually are going from one in 80 [for phase 1] to at least one in six [for phase 2], it suggests we’ve got a very good filter arrange. … There’s no cause to place sufferers in giant trials of unsafe and ineffective medicine. You wish to get rid of these medicine as early as attainable, and it looks like we do a fairly good job of that.”
‘Ethically justify the dangers’
The research knowledge strengthened Kimmelman’s view that part 2 research profit science greater than sufferers.
“Simply since you get a drug doesn’t imply you at all times profit from it,” he mentioned. “In lots of areas of medication, together with most cancers, you typically must deal with a number of sufferers with a drug for one affected person to really profit. Some most cancers immunotherapies have a quantity wanted to deal with of 1 in 4. Some most cancers medicine have a quantity wanted to deal with of 1 in 10. For those who put that variety of wanted to deal with subsequent to our numbers, it nonetheless reinforces that the prospect of really benefiting once you go right into a part 2 trial is fairly low.”
Kimmelman described scientific trials as “a human experiment.”
“The explanation we do scientific trials is to not ship remedy to sufferers,” he mentioned. “There isn’t actually good proof that it’s a really efficient technique to ship care to sufferers. The explanation we do scientific trials is to reply a scientific query. We’re asking sufferers to do us a favor and assist us reply a scientific query. We must have actually good science backing the research, and the research must be very nicely designed and applied from a scientific standpoint.”
Kimmelman has a number of concepts for increasing analysis into scientific trials so researchers might have extra knowledge to maximise total profit.
He and colleagues are evaluating the risk-benefit ratio of part 2 trials primarily based on tumor response and the possibilities of creating life-threatening toxicities. They added a part 3 management with that investigation.
Moreover, Kimmelman has a big curiosity in evaluating trial members’ final dream.
“When a whole lot of sufferers go into trials, they’re not eager about the common response sufferers have after they go into trials like this,” he mentioned. “What they’re eager about is, ‘If I am going into this trial, what’s the prospect of my profitable the lottery?’ … What’s the likelihood that should you go right into a part 1 trial that you simply’re to get entry to a drug like imatinib that extends your survival by 20 years. That’s such a vital query to reply for part 2 trials.”
Kimmelman additionally needs to understand how a lot info trials derive per affected person.
“It’s inconvenient to take part in a trial,” he mentioned. “You must make further clinic visits. You’ll have to expertise further biopsies and whatnot. You’re giving sufferers a drug that’s not confirmed protected or efficient. You actually wish to maximize how a lot info you get.”
Finally, these analysis concepts revolve round one query: “Whenever you run a trial, how do you ethically justify the dangers of giving a drug?” Kimmelman requested.
“I’ve at all times had the view that one of the best cause to take part in scientific trials is to create remedy alternatives for future sufferers,” he mentioned. “I nonetheless consider that. What this paper suggests is there’s a five-in-six likelihood you’re going to get a drug that’s not [ultimately] accepted. Some sufferers might imagine that these one-in-six odds of getting a drug that’s accepted are nonetheless favorable sufficient to wish to go right into a part 2 research. I’m not going to inform these sufferers how one can suppose. Our activity is to offer sufferers all the data we will and to assist them in making a very good choice.”
References:
For extra info:
Jonathan Kimmelman, PhD, may be reached at jonathan.kimmelman@mcgill.ca.





